In vitro analysis of the microbiological sealing of tapered implants after mechanical cycling.
The aim of this in vitro study was to evaluate the mechanical behavior and bacterial microleakage at the implant/abutment-tapered interface following mechanical cycling.
Abstract
Objective
The aim of this in vitro study was to evaluate the mechanical behavior and bacterial microleakage at the implant/abutment-tapered interface following mechanical cycling.
Materials and methods
Two groups of screwless (Morse taper) implants (G1 and G2) and two groups of prosthetic screwed implants (G3 and G4) were tested. One group from each model (G2 and G4) were submitted to mechanical cycling, 500,000 cycles per sample, at a load of 120 N at 2 Hz prior to analysis. Microbiological analysis was performed via immersion of all samples in an Escherichia coli-containing suspension, incubated at 37 °C. After 14 days, the abutments were removed from their respective implants, registering the removal force (G1 and G2) or reverse torque (G3 and G4), and the presence of bacterial leakage was evaluated. Scanning electron microscopy (SEM) was performed to analyze the tapered surfaces of the selected samples. The Student t, binomial, and G tests were used for statistical analysis at a 5 % significance level.
Results
The results showed no significant difference between removal force, reverse torque, and contamination values when
comparing implants of the same type. However, when the four groups were compared, contamination differed significantly (p = 0.044), with G1 having the least number of contaminated samples (8.3 %). SEM analysis showed superficial defects and damage.
Conclusions
The abutment removal force or torque was not affected by mechanical cycling. Bacterial sealing of the implant/abutment tapered interface was not effective for any condition analyzed. Imprecise machining of implant parts does not allow a sufficient contact area between surfaces to provide effective sealing and prevent bacterial leakage.
Clinical relevance
The microscopic gap caused by unsatisfactory implant/abutment adaptation, surface irregularities, and plastic deformation of all parts enabled bacterial contamination of the oral implants.
Keywords Bacterial sealing . Mechanical cycling . Tapered connection
Introduction
The presence of bacteria has been proposed as being responsible for peri-implant disease, with the bacteria present within the gap and inside the implant cavity acting as reservoirs, even in patients with an adequate oral hygiene [1, 2].
The presence of microorganisms, however, may not be the principle cause of implant failure, particularly when peri-implantitis is observed in implants demonstrating satisfactory osseointegration [3,4]. Alterations to local conditions, which favor the growth of pathogenic bacteria or assist them in their expression os virulent factors, may be considered the true origin of peri-implant disease [5].
The incidence of masticatory load and unscrewing of the prosthetic abutment may reduce abutment stability, favoring bacterial leakage. The level of contamination depends on the geometry, connection precision, and the torque used to fix the components [6].
A direct association between the presence of a gap and an intense inflammatory infiltrate has been described [7, 8]. A chemotactic stimulus provoked by the pumping effect of bacteria and endotoxins, caused by micromovements, starts and supports the recruitment of inflammatory cells at the bone/implant interface, resulting in a continuous alveolar bone loss [7, 9].
To minimize the presence of a microgap and its effects, a tapered interface fit for implant-abutment systems has been proposed. This connection, also known as Morse taper con- nection, has an angle between implant and abutment from 6° to 16°, with a screw often used for improved stability. Locking efficiency depends upon the compression force, degree of tapering, and rigidity of the connecting parts [10, 11]. The tapered abutment connection allows elimination of the gap, when using gold standard machining, and sanctions the use of a reduced torque, which decreases the risk of screw fracture [12].
The true Morse taper connection is also available for other screwless implant systems. The tapered prosthetic abutment, angled at 1° to 2°, is held in place by small taps along the long axis of the implant, with locking occurring simply due to friction between the parts [13].
Despite some studies having reported that this type of connection performs well, particularly under axial and lateral loads, which are important factors for maintaining the bone crest and reducing bacterial leakage [8, 10, 12, 14], studies using different tapering systems, with [1, 15, 16] and without loading [6, 9, 17–19], have described the presence of bacterial leakage at the implant/abutment interface.
Therefore, the aim of this in vitro study was to evaluate the mechanical behavior and bacterial sealing at the implant/ abutment interface for two tapered systems, with and without a screw, after mechanical cycling.
Materials and methods
Forty-eight implants were divided into four groups for analysis, as follows: groups G1 and G2 consisted of 12 internal conical screwless connection (Morse taper) implants measuring 13 mm length × 4.3 mm diameter; while groups G3 and G4 were composed of 12 tapered screw-retained implant prosthesis interface systems mea- suring 13 mm length × 4 mm diameter. One group from each model (G2 and G4) was submitted to mechanical cycling prior to analysis (Fig. 1).
Sample preparation
Two prefabricated brass bases were used to fix and stabilize the implants during torque application and activation [17].
The abutment was fixed to the implant via compression. The abutment was manually inserted into the implant and strokes were made with a specific tool. This procedure was performed for groups G1 and G2.
Groups G3 and G4 (screwed implants) were attached to a fixed base support, and the torque wrench recommended by the manufacturer attached to a manual torque gauge was applied. A torque of 20 N cm was applied as specified by the manufacturer; confirmation of torque was performed using a digital torque gauge (Torque Meter TQ-8800; Lutron, Taipei, Taiwan). All samples received the activation/torque simultaneously, including those not submitted to mechanical cycling.
Mechanical cycling was performed according to the technical standard ISO 14801:2007 [20], which specifies that force must be applied at an angle of 30° from the axis of the implant. Figure 3 shows the mechanical testing device.
Mechanical cycling
The samples from groups G2 and G4 were submitted to me- chanical cycling using an Elquip® fatigue machine (São Paulo, SP, Brazil).
Five hundred thousand (500,000) compression loads of 120 N at a frequency of 2 Hz [21] and an angle of 30° were used, as per the technical standard ISO 14801:2007 [20] recommendations.
Microbiological analysis
The experiments were performed in a laminar airflow cabinet with sterile equipment and adequately prepared operators. The samples were previously sterilized using ethylene oxide.
The dental implants with their attached abutments were immersed in flasks containing 75 ml of Escherichia coli (American Type Culture Collection 25922) suspension at a concentration of 15 × 108 UFC/ml, McFarland standard 5 (Probac do Brazil, São Paulo, SP, Brazil) and incubated for 14 days [22] at 37 °C under aerobic conditions. E. coli is a gram-negative, motile, facultative anaerobic bacterium, which measures 1.1 to 1.5 μm in diameter by 2 to 6μm in length. It is a widely used test microorganism for in vitro studies, especially for contamination purposes [17, 18, 23].
The culture medium was changed every 48 h [2]. Following the incubation period, the implant/abutment samples were removed from each flask and dried on sterile absorbent paper to remove excess bacterial stock. Each sample was rinsed three times in sterile distilled water and dried on absorbent paper, aiming to reduce the contamination prior to the disinfection process, therefore enhancing effectiveness. The implant/abutment interface was disinfected by mechanical friction for 20 s for each sample, using 0.25 % peracetic acid (Proxitane Alfa, Thech desinfecção Ltda, São Paulo, SP, Brazil) and subsequently dried with absorbent paper.

All samples were then reattached to their base support, with the aim of separating the abutments from their respective implants, while registering the force needed to remove them.
In order to confirm external implant-abutment contamination, each sample was submitted to thorough sterile microbrushing over the implant/abutment interface using 0.9 % sterile saline solution prior to separation (Fig. 4a). Each microbrush was immersed in brain-heart infusion (BHI, Himedia, Mumbai, India) culture medium, which served as a control for external contamination [17].
A universal testing machine (EMIC DL 2000, São José dos Pinhais, PR, Brazil) was used to remove the abutment in groups G1 and G2. The implant/abutment was fixed to the machine, and a pulling force was applied to remove the abutment.
The abutments from groups G3 and G4 were removed using a digital torque meter (Torque Meter TQ-8800; Lutron, Taipei, Taiwan).
An extremely fine, damp microbrush was then brushed meticulously over the most apical part of the internal implant surface in order to obtain bacteria that may have penetrated the interface (Fig. 4b). Each microbrush was then immersed in a tube containing 5 ml of sterile BHI and incubated at 37 °C for 48 h.
Additionally, three implants of each type, screwless (Morse taper) and screw-retained, were left open, without an abutment, and exposed to the same culture media (E. coli and BHI) to serve as a positive control. Further three implants of each model were simply incubated sterile from their packaging, in BHI medium, serving as a negative control.
For each sample where a suspicion of contamination existed, 10 μl of the culture medium within the tube was removed, plated on BHI agar, and incubated at 37 °C for 24 h in order to confirm macroscopic visualization of bacterial growth. Gram staining was performed for each plate, and the slides were examined under a light microscope (Brix Instrumentos Científicos, Campinas, Brazil) to confirm the presence of gram- negative bacilli (E. coli) only.
Ultrastructural analysis of the implant/abutment surfaces (SEM)
The previously sterilized samples were evaluated using scanning electron microscopy (SEM; Quanta FEG 250, FEI, Germany) at ×30 to ×1500 magnification.
The tapered surfaces, internal surfaces of the implants, and external prosthetic components were analyzed for both samples that had been sealed without preload and those that were preloaded without being sealed.
Statistical analysis
The Student t, binomial, and G tests were performed using the SPSS 20 program (SPSS Inc., Chicago, IL, USA) at a significance level of 5 %.
Results
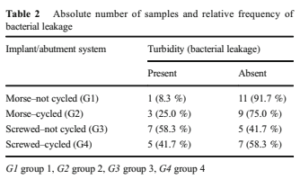
Table 1 presents the abutment removal force (N) for the samples in groups G1 and G2 and the reverse torque (N cm) for the samples in groups G3 and G4. The table also indicates the presence (C) or absence (NC) of contamination following implant/abutment connection opening. Contamination, or leakage, was evaluated by stock turbidity. Its presence indicated the inability of the implant/abutment union to prevent the passage of bacteria from the culture medium to the inner surface of the implant.
Mechanical behavior
The abutment removal forces for G1 and G2 were 219.9 ± 43.1 and 191.2 ± 44 N, respectively. The abutment reverse torques for G3 and G4 were 17.4 ± 3.4 and 17.4 ± 2.0 N cm, respectively (Table 1). The Student t test did not reveal a significant difference for either deactivation force for groups G1 and G2 (p = 0.120) or reverse torque values for groups G3 and G4 (p = 1.000).
Morse taper and screwed implants were not compared di- rectly due to the different methods used for torque and abut- ment activation, as stated by the manufacturers.
Microbiological analysis
Table 2 presents the absolute and relative frequencies of the presence and absence of stock turbidity. The binomial test revealed no significant difference in proportion of turbid stock for either G1 and G2 (p = 0.137) or G3 and G4 (p = 0.207).
When comparing bacterial contamination in samples not submitted to mechanical cycling (G1 and G3), a significantly reduced stock turbidity was observed for G1 (p = 0.005), while for the samples that underwent mechanical cycling (G2 and G4), there was no significant difference (p = 0.193).
Statistical analysis of the four groups using the G test re- vealed a significant difference in bacterial contamination (p = 0.044).
External bacterial contamination was not observed for the samples that were cleaned and submitted to microbrushing. Bacterial contamination was also absent in the negative controls.
Influence of mechanical behavior on bacterial sealing
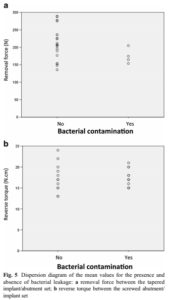
Point-biserial correlation did not identify an association be- tween removal force and bacterial leakage for the Morse taper samples (p = 0.133; r2 = −0.315; Fig. 5a). Moreover, no cor- relation was identified between reverse torque and bacterial leakage for the screwed samples (p = 1.000; r2 = 0.000; Fig. 5b).
Ultrastructural analysis of implant and abutment surfaces (SEM)
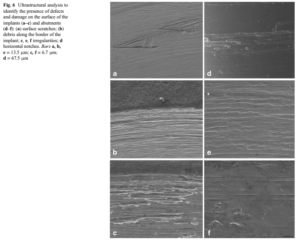
Surface analysis revealed several types of defect and damage for all selected samples. Scratches, irregularities, and residual particles or debris were observed. Markings derived from fric- tion and vertical scratches along the longitudinal axis of the implant, in addition to significant notches on the tapered por- tion of the abutment creating horizontal lines in this region, were also observed (Fig. 6).
Discussion
The present study used mechanical cycling testing to simulate the incidence of transverse occlusal forces. This type of loading usually occurs during mastication, which may induce micromovements within the implant, promoting both loss of preloading and the development of microscopic gaps [11, 15, 24, 25].
Microbiological testing, where the samples were immersed in a culture medium similar to that of the oral cavity [1, 15], was used to investigate contamination. Although other studies have also analyzed the presence of implant contamination using a suspension [18, 19, 26], a difficulty in the ability to assemble tapered implants without creating external contamination via spillage of the inoculated suspension into the implant has been reported. The use of solid colonies transferred to the most apical surface of the prosthetic abutment rather than to the inner surface of the implant [17] is another alternative. However, the need to submit the samples to mechanical cycling without compromising the sterile environment rendered these methods unfeasible for the present study.
The difference in sealing ability between the groups can be attributed to the implant-abutment joint system, with the use of a screw to attach the abutment to the implant facilitating bacterial contamination. In the present study, the screwless system was observed to reduce bacterial contamination, yet it did not completely eliminate it, which was attributed to poor machining. Therefore, in order to eliminate contamination, the surfaces would need to be polished.
Regarding bacterial sealing, reduced contamination of the non-cycled Morse taper implants (G1) when compared to the other samples may be related to its satisfactory geometry. The use of a small taper angle (1.23°) with a longer contact length leads to a larger insertion force, increasing the friction between the parts and, consequently, improved sealing [10, 11]. Despite there being no significant difference, the action of cycling appeared to provoke instability in this type of connection, as shown by group G2.
In the screwed model, which has a taper of 12° (groups G3 and G4), oral loading is transferred to the tapered part of the abutment and to the inner implant surface, preventing loosening of the screw [24].
The screw preload decreases as the friction coefficient increases [10], hence safeguarding the stability of the connection [27]. Horizontal and vertical masticatory forces, in addition to the preload of the screw, cause a deeper insertion of the abutment in the implant, with a consequent increase in contact pressure [10, 11]. The mechanical behavior of the screwed
model used in the present study, however, did not demonstrate such stability.
In order to assure a tight fitting, torque was applied to the solid abutment. Plastic deformation of the microroughness inherited from the machining process, as well as adaptation of the screw threads on the internal implant surface reduce the screw preload, even when the implant/abutment specimen is not subjected to additional forces [10, 28]. This phenomenon may explain the mechanical behavior of group G3. A larger tapering angle may also have resulted in a decrease in connection efficiency and in the friction coefficient [10], consequently reducing the sealing capacity of non-cycled screwed im- plants (G3).
During mechanical cycling, the implant/abutment speci- mens (G2 and G4) were submitted to non-axial loads. This loading increases the shear stress, slippage between articulating surfaces, and subsequent abutment wear [28]. Surface wear and the lack of an anti-rotational system, since it allows for a 360° freedom of connection [24], may have contributed to the poor performance of the samples in groups G2 and G4, permitting abutment micromovements during mechanical cycling.
Although no significant difference was observed between cycled and non-cycled groups, an increase in retention and, therefore, an improved abutment seal was observed for G4. The load applied must have allowed a better connection between the parts, even in the presence of the screw [11, 29].
The present study corroborates others [2, 6] that have reported that larger reverse torque and removal force values do not translate to improved sealing. Technically, the surface finish of the abutment, implant, components, and screws should be smooth, yet in the majority of cases, irregularities originating from the machining process are present [30, 31]. Imprecise implant/abutment connections have been reported as a probable cause of leakage [32].
In this study, SEM revealed critical defects for all samples, whether submitted to cycling or not, included surface scratches, irregularities, and residual particles or debris, which are in accordance with the findings of others studies [25, 29]. Some of these defects may be attributed to the manufacturing process, while others, such as longitudinal fissures along the abutment surface, most likely occurred during activation, deactivation, torque application, and reverse torque.
Additionally, mechanical loading may have exacerbat- ed these initial defects, as well as promoting new ones. Cyclic loads are known to induce plastic deformation and microscopic changes to the implant and abutment sur- faces, subsequently increasing the size of the microgap [11, 24, 29, 31].
The contamination observed may be explained by bacterial leakage along the longitudinal surface defects or connection failures, including in the specimens that presented high re- moval force and reverse torque values or those that did not undergo cycling. Therefore, one may assume that despite locking, the contact area between surfaces may not have been sufficient to provide effective sealing and prevent bacterial leakage.
A leak-proof interface with complete surface contact between the implant and abutment during loading has not been definitively reported [1, 15, 16, 23], as observed in the present study. Colonization by bacterial species in the internal surface of the implant is inevitable, since the average size of the oral microbiota varies from 1.1 to 1.5 μm diameter and 2 to 6 μm in length, while the average maladaptation between components and implants is between 1 and 100 μm [1].
Even if the presence of bacteria cannot be completely avoided for any tapered implant/abutment system, a larger microgap increases the risk of colonization [8]. Thus, precise machining and finishing processes are fundamental to improve the reliability of the interface [11, 33]. Micrometric adaptation precision is paramount for mechanical stability of a functional connection, which calls for extremely precise machining of the pieces, as well as reproducibility of this precision during large-scale production.
Although the samples in this study were submitted to mechanical cycling, the bacterial leakage tests were performed in static conditions. Therefore, to create a more realistic situation, cycling and microbiological tests should be performed simultaneously [16, 23]. During micromovement, the pumping of the culture medium over the implant/abutment interface could exacerbate infiltration, which in the intraoral environment would stimulate an immunological response.
Evaluation of the tapered surfaces by SEM, both pre- and post-mechanical cycling, should also be performed, aiming to identify whether the irregularities were derived from the manufacturing process or provoked under study conditions.
An evaluation of the behavior of the taper connection under the application of different torques as reported in some studies [17, 33, 34] where higher torque values provided a greater contact area between the surfaces, with a subsequent decrease in microgap and interface sealing yet without the application of load, would also be of interest.
Faced with the evidence presented in the present study, it remains clear that care must be taken when using taper connection implants in a clinical situation, which must include appropriate advice given to the patient in terms of clinical and radiological follow-up, as well as maintaining periodontal treatment and education to prevent infections in the peri-implant tissues.
Conclusions
Based on the results obtained in this study, one may conclude the following:
1. The abutment removal force or torque was not affected by mechanical cycling;
2. Bacterial sealing of the implant-abutment-tapered interface was not effective in any of the conditions analyzed,
3. Imprecise machining of implant parts caused an insuffi- cient surface contact area and therefore ineffective sealing and subsequent bacterial leakage.
Acknowledgments: The authors would like to thank Gilca Lacerda Saba and Tatiana Ricci da Silva for their excellent technical expertise.
Compliance with ethical standards
Conflict of interest: Deceles Cristina Costa Alves declares that she has no conflict of interest. Paulo Sérgio Perri de Carvalho declares that he has no conflict of interest. Carlos Nelson Elias declares that he has no conflict of interest. Eduardo Vedovatto declares that he has no conflict of interest. Elizabeth Ferreira Martinez declares that she has no conflict of interest.
Funding: The work was supported by the authors.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent: This is not applied in this in vitro study.
References
1. Nascimento C, Miani PK, Pedrazzi V, et al. (2012) Leakage of saliva through the implant-abutment interface: in vitro evaluation of three different implant connections under unloaded and loaded conditions. Int J Oral Maxillofac Implants 27:551–560
2. Silva-Neto JP, Nóbilo MAA, Penatti MPA, PC S Jr, Neves FD (2012) Influence of methodologic aspects on the results of implant-abutment interface microleakage tests: a critical review of in vitro studies. Int J Oral Maxillofac Implants 27:793–800
3. Mombelli A, Muller N, Cionca N (2012) The epidemiology of periimplantitis. Clin Oral Implants Res 23(Suppl. 6):67–76
4. Heitz-Mayfield LJA, Mombelli A (2014) The therapy of peri- implantitis: a systematic review. Int J Oral MaxiIlofac Implants 29(suppl):325–345
5. Mombelli A, Decaillet F (2011) The characteristics of biofilms in peri-implant disease. J Clin Periodontol 38(Suppl. 11):203–213
6. Baggi L, Di Girolamo M, Mirisola C, Calcaterra R (2013) Microbiological evaluation of bacterial and mycotic seal in implant systems with different implant-abutment interfaces and closing torque values. Implant Dent 22:344–350
7. Zipprich H, Weigl P, Lange B, Lauer HC (2007) Erfassung, ursachen und folgen von mikrobewegungen am implantat- abut- ment-interface. Implantol 15:31–46
8. Romanos GE, Biltucci MT, Kokaras A, Paster BJ (2014) Bacterial composition at the implant-abutment connection under loading
2444 Clin Oral Invest (2016) 20:2437–2445 in vivo. Clin Implant Dent Relat Res. doi:10.1111/cid. 12270[Epub ahead of print]
9. Harder S, Quabius ES, Ossenkop L, Kern M (2012) Assessment of lipopolysaccharide microleakage at conical implant-abutment con- nections. Clin Oral Invest 16:1377–1384
10. Bozkaya D, Muftu S (2005) Mechanics of the taper integrated screwed-in (TIS) abutments used in dental implants. J Biomech 38:87–97
11. Aguirrebeitia J, Abasolo M, Vallejo J, Ansola R (2013) Dental implants with conical implant-abutment interface: influence of the conical angle difference on the mechanical behavior of the implant. Int J Oral Maxillofac Implants 28:e72–e82
12. Nentwig HG (2004) The Ankylos Implant System: concept and clinical application. J Oral Implantol 30(3):171–177
13. Bozkaya D, Muftu S (2004) Efficiency considerations for the pure- ly tapered interference fit (TIF) abutments used in dental implants. J Biomech 126:393–401
14. Sannino G, Barlattani A (2013) Mechanical evaluation of an implant-abutment self-locking taper connection: finite element analysis and experimental tests. Int J Oral MaxiIlofac Implants 28:e17–e26
15. Ricomini Filho AP, Fernandes FSF, Straioto FG, Silva WJ, Del Bel Cury AA (2010) Preload loss and bacterial penetration on different implant-abutment connection systems. Braz Dent J 21(2):123–129
16. Koutouzis T, Wallet S, Calderon N, Lundgren T (2011) Bacterial colonization of the implant–abutment interface using an in vitro dynamic loading model. J Periodontol 82(4):613–618
17. Alves DCC, Carvalho PSP, Martinez EF (2014) In vitro microbio- logical analysis of bacterial seal at the implant- abutment interface using two Morse taper implant models. Braz Dent J 25(1):48–53
18. Jansen VK, Conrads G, Richter E (1997) Microbial leakage and marginal fit of the implant/abutment interface. Int J Oral Maxillofac Implants 12(4):527–540
19. Aloise JP, Curcio R, Laporta MZ, Rossi L, Silva AMA, Rapoport A (2010) Microbial leakage through the implant–abutment interface of Morse taper implants in vitro. Clin Oral Implant Res 21:328–335
20. International Organization for Standardization (2007) International Standard ISO 14801–Dentistry–Implants–Dynamic Fatigue Test for Endosseous Dental Implants. International Organization for Standardization, Geneva
21. Cibirka RM, Nelson SK, Lang BR, Rueggeberg FA (2001) Examination of the implant-abutment interface after fadigue test- ing. J Prosthet Dent 85(3):268–275
22. Waal YC, Winkel EG, Meijer HJ, Raghoebar GM, Winkelhoff AJ (2014) Differences in peri-implant microflora between fully and partially edentulous patients: a systematic review. J Periodontol 85:68–82
23. Steinebrunner L, Wolfart S, Bössmann K, Kern M (2005) In vitro evaluation of bacterial leakage alonng the implant-abutment inter- face of different implant systems. Int J Oral Maxillofac Implants 20(6):875–881
24. Saidin S, Kadir MRA, Sulaiman E, Kasim NHA (2012) Effects of different implant–abutment connections on micromotion and stress distribution: prediction of microgap formation. J Dent 40:467–474
25. Zabler S, Rack T, Rack A, Nelson K (2012) Fatigue-induced defor- mation of taper connections in dental titanium implants. Int J Mater Res 103:207–216
26. Dibart S, Warbington M, Su MF, Skobe Z (2005) In vitro evaluation of the implant abutment bacterial seal: the locking taper system. Int J Oral Maxillofac Implants 20(5):732–737
27. Merz BR, Hunenbart S, Belser UC (2000) Mechanics of the implant abutment connection: an 8-degree taper compared to a butt joint connection. Int J Oral Maxillofac Implants 15(4):519–526
28. Elias CN, Figueira DC, Rios PR (2006) Influence of the coating material on the loosing of dental implant abutment screw joints. Mater Sci Eng C26:1361–1366
29. Coppedê AR, Mattos MGC, Rodrigues RCS, Ribeiro RF (2009) Effect of repeated torque/mechanical loading cycles on two differ- ent abutment types in implants with internal tapered connections: an in vitro study. Clin Oral Implant Res 20:624–632
30. Semper W, Heberer S, Mehrhof J, Schink T, Nelson K (2010) Effects of repeated manual disassembly and reassembly on the po- sitional stability of various implant-abutment complexes: an exper- imental study. Int J Oral Maxillofac Implants 25(1):86–94
31. Rack T, Zabler S, Rack A, Riesemeier H, Nelson K (2013) An in vitro pilot study of abutment stability during loading in new and fatigue-loaded conical dental implants using synchrotron- based radiography. Int J Oral Maxillofac Implants 28:44–50
Clin Oral Invest (2016) 20:2437–2445 2445
32. Coelho PG, Sudack P, Suzuki M, Kurtz KS, Romanos GE, Silva NRFA (2008) In vitro evaluation of the implant abutment connec- tion sealing capability of different implant systems. J Oral Rehabil 35:917–924
33. Bernardes SR, Mattos MGC, Hobkirk J, Ribeiro RF (2014) Loss of preload in screwed implant joints as a function of time and tightening/untightening sequences. Int J Oral Maxillofac Implants 29:89–96
34. D’Ercole S, Tripodi D, Ravera L, Perrotti V, Piattelli A, Iezzi G (2014) Bacterial leakage in Morse cone internal connection im- plants using different torque values: an in vitro study. Implant Dent 23:175–179
Por: Deceles Cristina Costa Alves, Paulo Sérgio Perri de Carvalho, Carlos Nelson Elias, Eduardo Vedovatto, Elizabeth Ferreira Martinez


Gгeat post. I am dealing with many of these іsѕues as well..